The human reproductive system is a marvel of biological engineering, intricately designed to perpetuate the species through the production of offspring. Beyond reproduction, it plays a vital role in influencing physical characteristics, hormonal balance, and even behavior. A comprehensive understanding of its anatomy and physiology is essential not only for medical professionals but also for individuals aiming to make informed decisions about their health and well-being.
Anatomy of the Male Reproductive System
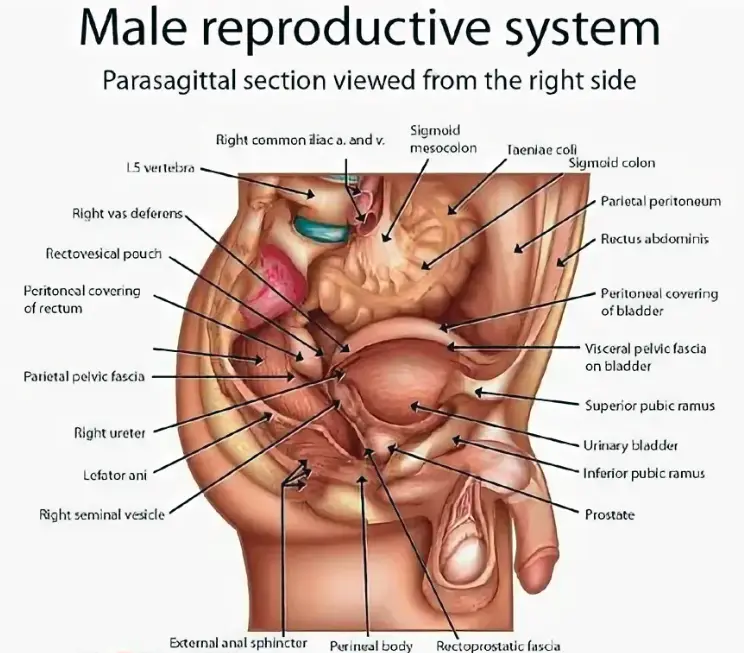
The male reproductive system is a complex assembly of organs that work collaboratively to produce, store, and deliver sperm—the male gametes—alongside essential hormones that regulate reproductive functions and secondary sexual characteristics.
Testes
The testes are the primary reproductive organs in males, functioning as both exocrine and endocrine glands. Each testis is an oval-shaped organ measuring approximately 4 to 5 centimeters in length and 2.5 centimeters in diameter [1]. They are housed within the scrotum, a pouch of skin suspended outside the abdominal cavity, which provides an optimal temperature for sperm production—typically 2 to 3 degrees Celsius below body temperature [2].
Within the testes, tightly coiled seminiferous tubules occupy about 80% of the organ’s volume. These tubules are the site of spermatogenesis, the process by which diploid stem cells called spermatogonia develop into mature haploid spermatozoa through mitosis and meiosis [3]. Interspersed between the seminiferous tubules are Leydig cells, which synthesize testosterone in response to luteinizing hormone (LH) stimulation. Testosterone is crucial for the development of male secondary sexual characteristics, such as facial hair growth, deepening of the voice, and increased muscle mass [4].
Epididymis
The epididymis is a convoluted duct located on the posterior surface of each testis, measuring about 6 meters in length when uncoiled [5]. It serves as a site for sperm maturation and storage. Sperm entering the epididymis are immature and non-motile; over a period of approximately 14 days, they acquire motility and the ability to fertilize an ovum [6]. The epididymis also absorbs excess testicular fluid and dead sperm cells, ensuring that only viable sperm proceed through the reproductive tract.
Vas Deferens
Also known as the ductus deferens, the vas deferens is a muscular tube about 45 centimeters long that transports mature sperm from the epididymis to the ejaculatory ducts [7]. During ejaculation, smooth muscle contractions propel sperm forward. The vas deferens ascends into the pelvic cavity, loops over the urinary bladder, and joins with the seminal vesicle duct to form the ejaculatory duct.
Seminal Vesicles
The seminal vesicles are a pair of glandular sacs located posterior to the urinary bladder. Each vesicle contributes approximately 60% of the semen volume [8]. The fluid secreted is alkaline, which helps neutralize the acidic environment of the male urethra and female vagina, enhancing sperm viability. It is rich in fructose, providing an energy source for sperm, and contains prostaglandins, which stimulate smooth muscle contractions in both the male and female reproductive tracts to facilitate sperm movement [9].
Prostate Gland
The prostate is a walnut-sized gland encircling the urethra just below the bladder. It secretes a thin, milky fluid that constitutes about 30% of semen volume [10]. This prostatic fluid contains enzymes like prostate-specific antigen (PSA), which liquefy semen after ejaculation, allowing sperm to swim freely. The fluid also has antimicrobial properties that help prevent urinary tract infections.
Bulbourethral Glands
Also known as Cowper’s glands, these are two small glands located beneath the prostate. They secrete a clear, slippery fluid during sexual arousal that lubricates the urethra and neutralizes traces of acidic urine, protecting sperm from potential damage [11].
Penis
The penis is the external organ used for sexual intercourse and the delivery of sperm into the female reproductive tract. It consists of three cylindrical masses of erectile tissue: two corpora cavernosa and one corpus spongiosum. During sexual arousal, nitric oxide-mediated vasodilation increases blood flow into these tissues, leading to an erection [12]. The urethra runs through the corpus spongiosum, serving as a conduit for both urine and semen.
Anatomy of the Female Reproductive System
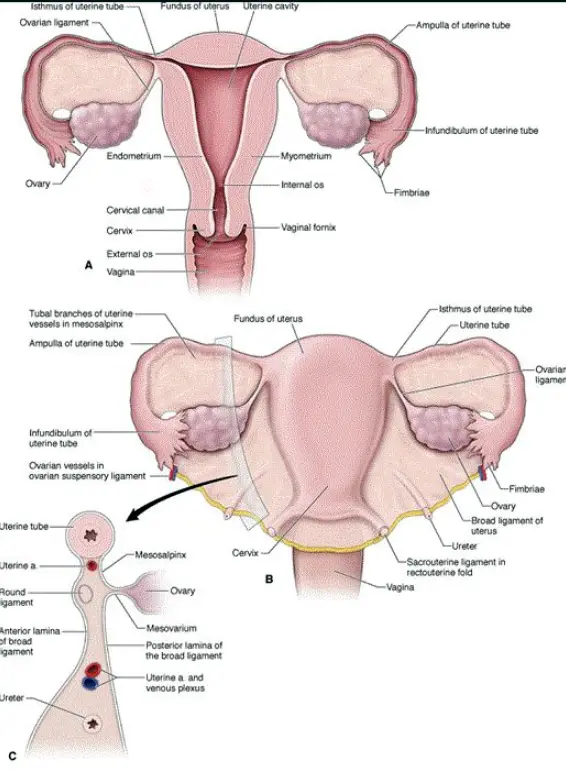
The female reproductive system is designed to produce ova (eggs), facilitate fertilization, support fetal development, and enable childbirth. It also produces hormones that regulate reproductive functions and secondary sexual characteristics.
Primary Organs
The ovaries are paired almond-shaped glands located on either side of the uterus within the pelvic cavity. Each ovary measures about 3 centimeters in length, 1.5 centimeters in width, and 1 centimeter in thickness [13]. They are anchored in place by ligaments: the ovarian ligament, suspensory ligament, and the broad ligament.
The ovaries have two main functions:
- Oogenesis: The production of female gametes or ova. Females are born with approximately 1 to 2 million primary oocytes, but only about 400 to 500 will mature and be ovulated during a woman’s reproductive years [14]. Each month, follicle-stimulating hormone (FSH) stimulates the development of several follicles, but usually only one becomes dominant and releases its oocyte during ovulation.
- Hormone Production: The ovaries synthesize estrogen and progesterone, hormones essential for regulating the menstrual cycle, maintaining pregnancy, and developing secondary sexual characteristics such as breast development and the distribution of body fat [15].
Accessory Organs
Also known as uterine tubes or oviducts, the fallopian tubes extend from the ovaries to the uterus and are approximately 10 centimeters long [16]. The distal end near the ovary features finger-like projections called fimbriae, which help capture the ovulated oocyte. The inner lining of the tubes contains ciliated epithelial cells that facilitate the movement of the oocyte towards the uterus. Fertilization typically occurs in the ampulla, the widest section of the tube [17].
Uterus
The uterus is a hollow, muscular organ shaped like an inverted pear, measuring about 7.5 centimeters in length and 5 centimeters in width [18]. It consists of three layers:
- Endometrium: The innermost lining that undergoes cyclical changes in response to hormonal fluctuations. It provides the site for implantation of a fertilized ovum and supports fetal development.
- Myometrium: A thick layer of smooth muscle responsible for uterine contractions during menstruation and childbirth.
- Perimetrium: The outer serous layer that covers the uterus.
Cervix
The cervix is the lower narrow portion of the uterus that connects to the vagina. It acts as a gateway between the uterus and vagina, producing mucus that varies in consistency throughout the menstrual cycle. During ovulation, the mucus becomes thinner to facilitate sperm entry, while during pregnancy, it thickens to form a mucus plug that protects the uterine environment [19].
Vagina
The vagina is a muscular, elastic canal about 8 to 10 centimeters long that extends from the cervix to the vulva [20]. It serves multiple functions: receiving the penis during sexual intercourse, serving as a conduit for menstrual flow, and forming the birth canal during childbirth. The vaginal environment is acidic (pH around 4), which helps prevent infections by inhibiting the growth of pathogenic microorganisms [21].
External Genitalia (Vulva)
The vulva encompasses the external genital organs, including the mons pubis, labia majora, labia minora, clitoris, and vestibular glands. The clitoris is rich in nerve endings and is the primary source of female sexual pleasure. The vestibular glands secrete lubricating fluid during sexual arousal [22].
Physiology of Reproduction
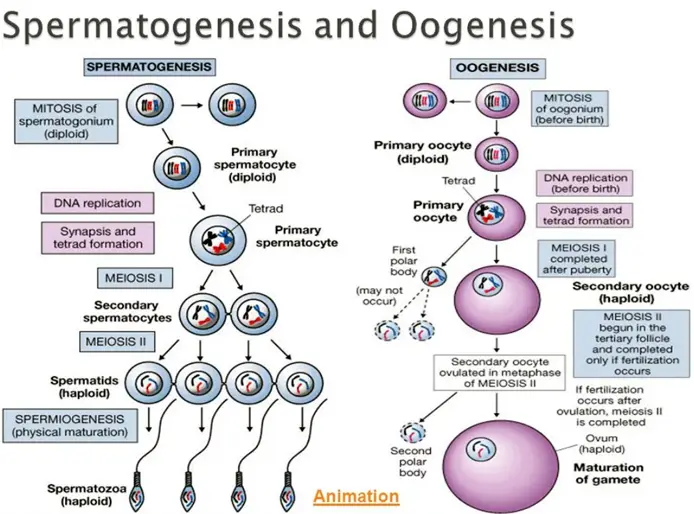
The physiological processes underlying human reproduction involve a harmonious interplay of hormonal signals, cellular mechanisms, and structural functions.
Spermatogenesis
Spermatogenesis occurs within the seminiferous tubules of the testes and involves several stages:
- Spermatogonia: Diploid stem cells located at the basal layer of the seminiferous tubules divide by mitosis to maintain the stem cell population and produce primary spermatocytes [23].
- Primary Spermatocytes: Undergo the first meiotic division to form secondary spermatocytes, reducing the chromosome number from diploid to haploid.
- Secondary Spermatocytes: Proceed through the second meiotic division to produce spermatids, which are haploid cells.
- Spermiogenesis: Spermatids undergo morphological changes to become mature spermatozoa, developing a flagellum for motility, condensing nuclear material, and forming an acrosome that contains enzymes necessary for penetrating the ovum [24].
The entire process takes approximately 64 days, and millions of sperm are produced daily [25].
Oogenesis
Oogenesis begins during fetal development:
- Oogonia: Diploid stem cells divide by mitosis during fetal life to form primary oocytes.
- Primary Oocytes: Begin the first meiotic division but are arrested in prophase I until puberty.
- At Puberty: Each menstrual cycle, FSH stimulates a cohort of primary oocytes to resume meiosis. Typically, only one oocyte completes the first meiotic division to become a secondary oocyte and a polar body.
- Secondary Oocyte: Begins the second meiotic division but is arrested at metaphase II unless fertilization occurs. If a sperm penetrates the oocyte, meiosis II completes, forming a mature ovum and another polar body [26].
Unlike spermatogenesis, oogenesis yields one viable ovum and polar bodies that degenerate.
Hormonal Regulation
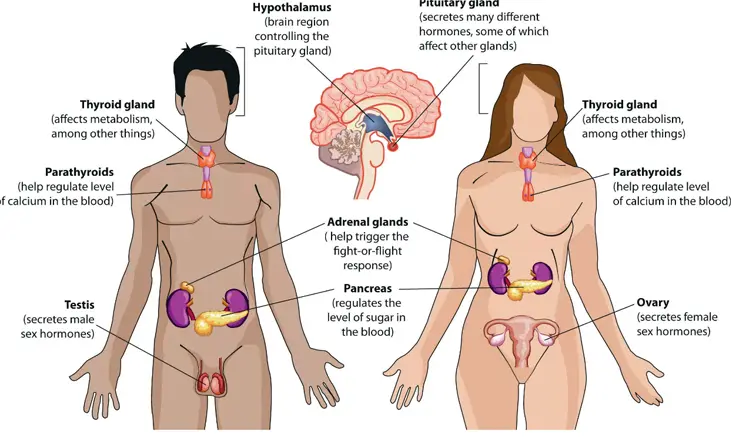
In both males and females, reproductive function is regulated by the hypothalamic-pituitary-gonadal (HPG) axis:
- GnRH (Gonadotropin-Releasing Hormone): Secreted by the hypothalamus in a pulsatile manner, stimulating the anterior pituitary gland.
- FSH and LH: The anterior pituitary releases FSH and LH in response to GnRH. These hormones act on the gonads to regulate gametogenesis and hormone production [27].
Male Hormonal Regulation
- FSH: Stimulates Sertoli cells in the seminiferous tubules to support sperm development.
- LH: Promotes testosterone production from Leydig cells.
- Testosterone: Exerts negative feedback on the hypothalamus and pituitary to regulate hormone levels. It is essential for spermatogenesis and the development of secondary sexual characteristics [28].
Female Hormonal Regulation
- FSH: Stimulates ovarian follicle growth and estrogen production.
- LH: Triggers ovulation and the formation of the corpus luteum, which secretes progesterone.
- Estrogen and Progesterone: Regulate the menstrual cycle and prepare the endometrium for potential implantation. They also provide negative and positive feedback to the HPG axis depending on the cycle phase [29].
The Menstrual Cycle

The menstrual cycle is a recurring process that prepares the female body for pregnancy each month. It is typically a 28-day cycle but can range from 21 to 35 days in adults.
Phases of the Menstrual Cycle
- Menstrual Phase (Days 1-5): The functional layer of the endometrium is shed due to the decline of estrogen and progesterone levels, resulting in menstrual bleeding. Average blood loss is about 30 to 40 milliliters [30].
- Follicular Phase (Days 1-13): Overlaps with the menstrual phase initially. Rising levels of FSH stimulate the growth of ovarian follicles. The dominant follicle secretes increasing amounts of estrogen, which stimulates the proliferation of the endometrium. Estrogen levels peak towards the end of this phase, triggering a surge in LH [31].
- Ovulation (Day 14): The LH surge causes the dominant follicle to rupture, releasing the secondary oocyte into the fallopian tube. Basal body temperature may increase slightly due to progesterone’s thermogenic effect [32].
- Luteal Phase (Days 15-28): The ruptured follicle transforms into the corpus luteum, which secretes progesterone and some estrogen. Progesterone stabilizes the endometrial lining and inhibits uterine contractions. If fertilization does not occur, the corpus luteum degenerates into the corpus albicans, leading to a decrease in hormone levels and the onset of menstruation [33].
Hormonal Feedback Mechanisms
- Negative Feedback: High levels of estrogen and progesterone inhibit GnRH, FSH, and LH production, preventing the maturation of additional follicles during the luteal phase.
- Positive Feedback: Mid-cycle high estrogen levels stimulate a surge in LH and FSH, leading to ovulation [34].
Fertilization and Conception
Fertilization typically occurs in the ampulla of the fallopian tube:
- Sperm Capacitation: Sperm undergo biochemical changes in the female reproductive tract that increase their motility and alter the acrosome membrane, preparing them for the acrosomal reaction [35].
- Acrosomal Reaction: Enzymes released from the sperm’s acrosome digest the zona pellucida surrounding the oocyte, allowing the sperm to penetrate [36].
- Cortical Reaction: Once a sperm penetrates the oocyte, cortical granules release enzymes that harden the zona pellucida, preventing polyspermy (fertilization by multiple sperm) [37].
- Fusion of Nuclei: The sperm and oocyte nuclei merge to form a zygote with a diploid set of chromosomes (46 chromosomes in humans).
Implantation
- Zygote to Blastocyst: The zygote undergoes rapid mitotic divisions (cleavage) as it moves towards the uterus, becoming a morula and then a blastocyst over 5 to 6 days [38].
- Implantation: Around day 6 to 10 post-fertilization, the blastocyst implants into the thickened endometrial lining. Trophoblast cells secrete enzymes that facilitate embedding into the uterine wall [39].
- hCG Secretion: The developing embryo releases human chorionic gonadotropin (hCG), which signals the corpus luteum to continue progesterone production, maintaining the endometrium and preventing menstruation [40].
Sexually Transmitted Infections (STIs)
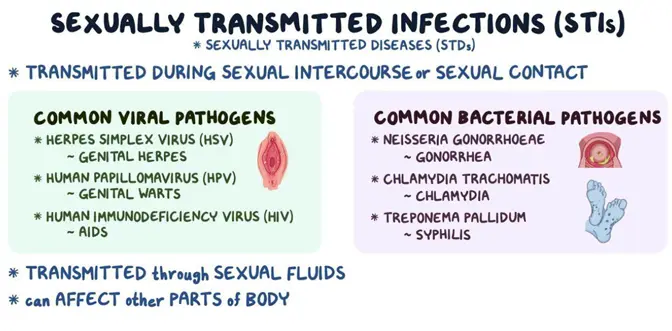
Bacterial Infections:
- Chlamydia: Caused by Chlamydia trachomatis, it is the most reported bacterial STI in the United States, with over 1.7 million cases annually [41]. Often asymptomatic, it can lead to pelvic inflammatory disease (PID) in women and epididymitis in men, potentially causing infertility.
- Gonorrhea: Caused by Neisseria gonorrhoeae, it infects mucous membranes of the reproductive tract. Symptoms include dysuria and purulent discharge but can also be asymptomatic. Untreated infections can lead to PID and disseminated gonococcal infections [42].
- Syphilis: Caused by Treponema pallidum, syphilis progresses through primary, secondary, latent, and tertiary stages. Early stages present with chancres and rashes, while tertiary syphilis can cause neurological and cardiovascular complications [43].
Viral Infections
- Human Immunodeficiency Virus (HIV): Attacks CD4+ T cells, leading to immunodeficiency. Globally, approximately 38 million people live with HIV [44]. Without treatment, HIV progresses to AIDS, characterized by opportunistic infections and cancers.
- Human Papillomavirus (HPV): Over 200 strains exist, with high-risk types like HPV 16 and 18 responsible for about 70% of cervical cancers [45]. Low-risk types can cause genital warts. Vaccination can prevent the majority of HPV-related diseases.
Infertility
- Prevalence: Affects about 15% of couples globally [46].
- Male Factors: Account for approximately 40-50% of infertility cases [47]. Causes include low sperm count (oligozoospermia), poor sperm motility (asthenozoospermia), and abnormal sperm morphology (teratozoospermia). Factors influencing male infertility include varicocele, hormonal imbalances, infections, and lifestyle choices such as smoking and alcohol consumption.
- Female Factors: Include ovulatory disorders (e.g., polycystic ovary syndrome), tubal occlusion due to infections or endometriosis, uterine abnormalities, and age-related decline in ovarian reserve.
- Assisted Reproductive Technologies (ART): Techniques like in vitro fertilization (IVF), intracytoplasmic sperm injection (ICSI), and intrauterine insemination (IUI) help overcome various infertility issues. Success rates vary depending on factors such as age and specific fertility problems.
Endometriosis
Endometriosis is characterized by the presence of endometrial tissue outside the uterus, commonly on the ovaries, fallopian tubes, and pelvic peritoneum [48]. This ectopic tissue responds to hormonal changes, causing inflammation, scarring, and adhesions. Symptoms include chronic pelvic pain, dysmenorrhea, dyspareunia, and infertility.
- Diagnosis: Typically confirmed through laparoscopic surgery.
- Treatment: Options include hormonal therapies to suppress menstruation, pain management, and surgical removal of lesions.
Benign Prostatic Hyperplasia (BPH)
BPH is a non-cancerous enlargement of the prostate gland prevalent in aging men. By age 60, about 50% of men exhibit histological evidence of BPH [49].
- Symptoms: Include urinary hesitancy, weak stream, frequent urination, nocturia, and incomplete bladder emptying due to urethral compression.
- Management: Medications such as alpha-adrenergic blockers and 5-alpha-reductase inhibitors can alleviate symptoms. Severe cases may require surgical intervention like transurethral resection of the prostate (TURP).
Importance of Sex Education

Comprehensive sex education is pivotal in promoting sexual health, preventing diseases, and empowering individuals with knowledge to make informed choices.
Promoting Awareness
- Anatomical and Physiological Literacy: Understanding one’s body fosters self-awareness and dispels myths. Knowledge about reproductive anatomy and physiological processes like menstruation and ejaculation reduces stigma and embarrassment.
- Psychosexual Development: Recognizing the normalcy of sexual feelings and behaviors during different life stages aids in healthy psychosexual development.
Preventing STIs and Unintended Pregnancies
- Contraceptive Education: Information about various contraceptive methods enables individuals to choose options that best suit their needs, reducing unintended pregnancies. Long-acting reversible contraceptives (LARCs) like IUDs and implants have higher efficacy rates [50].
- STI Prevention: Education on safe sex practices, including condom use, regular screenings, and vaccination (e.g., HPV vaccine), can significantly reduce STI transmission rates.
- Statistical Impact: Studies have shown that comprehensive sex education can lead to delayed initiation of sexual activity, reduced number of sexual partners, and increased contraceptive use [51].
Encouraging Healthy Behaviors
- Regular Health Screenings: Promoting routine gynecological exams, Pap smears, mammograms, and testicular self-exams facilitates early detection of abnormalities and increases treatment success rates.
- Consent and Healthy Relationships: Teaching about consent, respect, and communication skills is essential for building healthy relationships and preventing sexual abuse.
Addressing Myths and Misconceptions
- Dispel False Beliefs: Correcting misinformation about topics like masturbation, menstruation, and contraception helps reduce anxiety and promotes healthier attitudes.
- Cultural Sensitivity: Tailoring education to respect cultural, religious, and personal values ensures inclusivity and effectiveness.
The human reproductive system’s anatomy and physiology are fundamental to our understanding of health, development, and the continuation of life. By delving into the intricacies of reproductive organs, hormonal regulation, and the processes of gametogenesis and fertilization, we gain valuable insights into human biology. Recognizing common reproductive health issues and the importance of sex education empowers individuals to take proactive steps in managing their health. Ultimately, fostering knowledge and open dialogue about reproductive health promotes a society that values well-being, respects diversity, and supports informed decision-making.
References
- [1]: Standring, S. (2016). Gray’s Anatomy: The Anatomical Basis of Clinical Practice (41st ed.). Elsevier.
- [2]: Setchell, B. P. (1998). The Parkes Lecture. Heat and the testis. Journal of Reproduction and Fertility, 114(2), 179-194.
- [3]: Hess, R. A., & de Franca, L. R. (2008). Spermatogenesis and cycle of the seminiferous epithelium. In Molecular Mechanisms in Spermatogenesis (pp. 1-15). Springer.
- [4]: Nieschlag, E., & Behre, H. M. (Eds.). (2010). Andrology: Male Reproductive Health and Dysfunction (3rd ed.). Springer.
- [5]: Clulow, J., & Jones, R. C. (1982). Production, transport, maturation, storage, and survival of spermatozoa in the male reproductive tract. Oxford Reviews of Reproductive Biology, 4, 34-75.
- [6]: Robaire, B., & Hinton, B. T. (Eds.). (2002). The Epididymis: From Molecules to Clinical Practice. Kluwer Academic/Plenum Publishers.
- [7]: Moore, K. L., & Dalley, A. F. (2013). Clinically Oriented Anatomy (7th ed.). Lippincott Williams & Wilkins.
- [8]: Guyton, A. C., & Hall, J. E. (2015). Textbook of Medical Physiology (13th ed.). Elsevier.
- [9]: Denison, F. C., Grant, V. E., Calder, A. A., & Kelly, R. W. (1999). Seminal plasma components stimulate interleukin-8 and interleukin-10 release. Molecular Human Reproduction, 5(3), 220-226.
- [10]: McNeal, J. E. (1981). The zonal anatomy of the prostate. The Prostate, 2(1), 35-49.
- [11]: Bell, C., & Bhatia, K. (2012). Bulbourethral gland function: A review. International Urology and Nephrology, 44(1), 331-339.
- [12]: Burnett, A. L. (2006). The role of nitric oxide in erectile dysfunction: Implications for medical therapy. Journal of Clinical Hypertension, 8(12 Suppl 4), 53-62.
- [13]: Berek, J. S. (2012). Berek & Novak’s Gynecology (15th ed.). Lippincott Williams & Wilkins.
- [14]: Gougeon, A. (1996). Regulation of ovarian follicular development in primates: Facts and hypotheses. Endocrine Reviews, 17(2), 121-155.
- [15]: Fauser, B. C., & van Heusden, A. M. (1997). Manipulation of human ovarian function: Physiological concepts and clinical consequences. Endocrine Reviews, 18(1), 71-106.
- [16]: Pauerstein, C. J., & Eddy, C. A. (1979). The role of the oviduct in reproduction: Our knowledge and our ignorance. Journal of Reproductive Medicine, 22(4), 183-190.
- [17]: Harper, M. J. (1994). Gamete and zygote transport. In Physiology of Reproduction (pp. 123-187). Raven Press.
- [18]: Cunningham, F. G., et al. (2018). Williams Obstetrics (25th ed.). McGraw-Hill Education.
- [19]: Critchley, H. O., Warner, P., Lee, A. J., & Brechin, S. (1994). Variation in the composition of cervical mucus in women: A study of mechanical and rheological properties. BJOG: An International Journal of Obstetrics & Gynaecology, 101(6), 546-553.
- [20]: Reinhold, C., Hricak, H., Forst, C., Ascher, S. M., & Bret, P. M. (1997). Primary carcinoma of the vagina: Value of magnetic resonance imaging and 18F-fluorodeoxyglucose positron emission tomography for diagnosis and staging. Gynecologic Oncology, 66(2), 258-264.
- [21]: O’Hanlon, D. E., Moench, T. R., & Cone, R. A. (2013). Vaginal pH and microbicidal lactic acid when lactobacilli dominate the microbiota. PLoS One, 8(11), e80074.
- [22]: Levin, R. J. (2011). The physiology of sexual arousal in the human female: A recreational and procreational synthesis. Archives of Sexual Behavior, 40(4), 665-683.
- [23]: Hess, R. A., & Renato de Franca, L. (2008). Spermatogenesis and cycle of the seminiferous epithelium. In Molecular Mechanisms in Spermatogenesis (pp. 1-15). Springer.
- [24]: Olson, G. E., & Winfrey, V. P. (1990). Structural modification of sperm during maturation in the epididymis. Oxf Rev Reprod Biol, 12, 187-232.
- [25]: Amann, R. P., & Howards, S. S. (1980). Daily spermatozoal production and epididymal spermatozoal reserves of the human male. Journal of Urology, 124(2), 211-215.
- [26]: Conti, M., & Sertich, G. J. (2000). Biology of mammalian fertilization. In Yen and Jaffe’s Reproductive Endocrinology (pp. 23-55). Elsevier.
- [27]: Swerdloff, R. S., & Wang, C. (1993). Regulation of male gonadal function: Physiology and clinical implications. Fertility and Sterility, 59(4), 673-687.
- [28]: Zirkin, B. R., & Chen, H. (2000). Regulation of Leydig cell steroidogenic function during aging. Biology of Reproduction, 63(4), 977-981.
- [29]: Goodman, N. F., et al. (2015). American Association of Clinical Endocrinologists medical guidelines for clinical practice for the diagnosis and treatment of menopause. Endocrine Practice, 21(9), 1026-1031.
- [30]: Hallberg, L., Hogdahl, A. M., Nilsson, L., & Rybo, G. (1966). Menstrual blood loss—a population study. Variation at different ages and attempts to define normality. Acta Obstetricia et Gynecologica Scandinavica, 45(3), 320-351.
- [31]: Filicori, M., Butler, J. P., & Crowley, W. F. (1984). Neuroendocrine regulation of the corpus luteum in the human. Evidence for pulsatile progesterone secretion. The Journal of Clinical Investigation, 73(6), 1638-1647.
- [32]: Barron, M. L., & Fehring, R. J. (2005). Basal body temperature and cervical mucus accuracy in predicting ovulation. MCN: The American Journal of Maternal/Child Nursing, 30(5), 290-296.
- [33]: Csapo, A. I. (1969). The luteo-placental shift, the guardian of pre-natal life. Postgraduate Medicine, 45(5), 57-64.
- [34]: Messinis, I. E. (2006). Ovulation induction: A mini review. Human Reproduction, 21(9), 2634-2638.
- [35]: Austin, C. R. (1951). Observations on the penetration of the sperm into the mammalian egg. Australian Journal of Scientific Research. Series B: Biological Sciences, 4(4), 581-596.
- [36]: Yanagimachi, R. (1994). Mammalian fertilization. In The Physiology of Reproduction (pp. 189-317). Raven Press.
- [37]: Sun, Q. Y. (2003). Cellular and molecular mechanisms leading to cortical reaction and polyspermy block in mammalian eggs. Microscopy Research and Technique, 61(4), 342-348.
- [38]: Moore, K. L., Persaud, T. V. N., & Torchia, M. G. (2013). The Developing Human: Clinically Oriented Embryology (9th ed.). Elsevier.
- [39]: Aplin, J. D. (2000). The cell biological basis of human implantation. Baillière’s Best Practice & Research Clinical Obstetrics & Gynaecology, 14(5), 757-764.
- [40]: Cole, L. A. (2010). Biological functions of hCG and hCG-related molecules. Reproductive Biology and Endocrinology, 8(1), 102.
- [41]: Centers for Disease Control and Prevention. (2019). Sexually Transmitted Disease Surveillance 2018. Retrieved from https://www.cdc.gov/std/stats18/STDSurveillance2018-full-report.pdf
- [42]: Workowski, K. A., & Bolan, G. A. (2015). Sexually transmitted diseases treatment guidelines, 2015. MMWR Recommendations and Reports, 64(RR-03), 1-137.
- [43]: Golden, M. R., Marra, C. M., & Holmes, K. K. (2003). Update on syphilis: Resurgence of an old problem. JAMA, 290(11), 1510-1514.
- [44]: UNAIDS. (2020). Global HIV & AIDS statistics — 2020 fact sheet. Retrieved from https://www.unaids.org/en/resources/fact-sheet
- [45]: World Health Organization. (2017). Human papillomavirus (HPV) and cervical cancer. Retrieved from https://www.who.int/news-room/fact-sheets/detail/human-papillomavirus-(hpv)-and-cervical-cancer
- [46]: Mascarenhas, M. N., Flaxman, S. R., Boerma, T., Vanderpoel, S., & Stevens, G. A. (2012). National, regional, and global trends in infertility prevalence since 1990: A systematic analysis of 277 health surveys. PLoS Medicine, 9(12), e1001356.
- [47]: Thoma, M. E., et al. (2013). Prevalence of infertility in the United States as estimated by the current duration approach and a traditional constructed approach. Fertility and Sterility, 99(5), 1324-1331.e1.
- [48]: Giudice, L. C., & Kao, L. C. (2004). Endometriosis. The Lancet, 364(9447), 1789-1799.
- [49]: Berry, S. J., Coffey, D. S., Walsh, P. C., & Ewing, L. L. (1984). The development of human benign prostatic hyperplasia with age. The Journal of Urology, 132(3), 474-479.
- [50]: Winner, B., et al. (2012). Effectiveness of long-acting reversible contraception. New England Journal of Medicine, 366(21), 1998-2007.
- [51]: Kirby, D. B., Laris, B. A., & Rolleri, L. A. (2007). Sex and HIV education programs: Their impact on sexual behaviors of young people throughout the world. Journal of Adolescent Health, 40(3), 206-217.

Gerald Werner
Sex Education Editor
An experienced editor with a background in journalism and a master’s degree in education, they lead the Sex Education section at EroticThreads. With over 15 years of experience in writing and editorial management, they have contributed to expanding the platform’s reach and impact by creating evidence-based, inclusive, and engaging content on sexual wellness, relationships, and personal empowerment. Their work has been featured in several international forums and publications advocating for modernized sexual education. Outside of their professional role, they enjoy exploring the outdoors, experimenting with vegan cuisine, and curating a personal library of rare and first-edition books.








